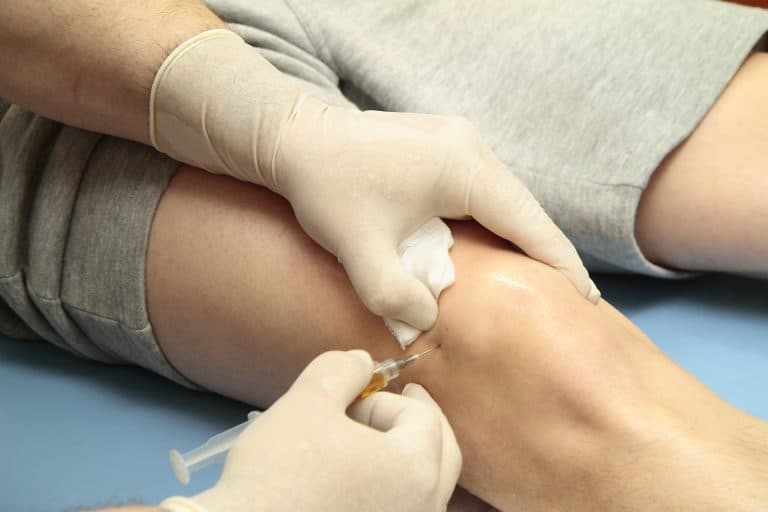
Arthrosamid® Safety, Complications & Context
An open and balanced discussion regarding the long-term clinical profile of polyacrylamide hydrogel (PAAG) treatments.
Arthrosamid® (a registered trademark of Contura A/S.).
All medical treatments carry potential risks. This includes Arthrosamid®, a form of polyacrylamide hydrogel (PAAG).
While Arthrosamid® shows favourable short-to-medium term safety for knee osteoarthritis, patients deserve full awareness of complications observed in other medical contexts.
What Has Been Reported in the Medical Literature
There are published papers describing complications related to PAAG, most notably under the brand name Aquamid (a chemically similar PAAG product from the same manufacturer group, marketed under a different label).
These reports primarily relate to:
- Cosmetic and soft-tissue injections
- Large injection volumes
- Non-joint tissues (breast, fat)
- Long-term follow-up (many years)
Reported complications include:
- Chronic inflammation
- Infection (delayed presentations)
- Nodules or firmness
- Migration of material
- Need for surgical removal
For transparency, patients who wish to explore this literature in detail are encouraged to read the published review article on PAAG (Aquamid) complications.

Why Context Matters
It is important to understand that clinical outcomes are heavily influenced by the environment and method of application:
The knee joint is a very different biological environment from soft tissue.
Arthrosamid® for knee osteoarthritis is delivered:
- In much smaller volumes
- Inside the joint, not under the skin
- Using image-guided techniques
Most published complication data relate to historic cosmetic use, not modern intra-articular knee treatment.
"That said, differences in indication do not eliminate risk, and this literature reinforces why careful patient selection, technique, and follow-up are essential."

What We See in Knee
Osteoarthritis Practice
In our own independent clinical study and routine practice:
Complications following intra-articular PAAG injection have generally been uncommon and usually mild.
The most frequently observed issues are:
Serious joint infection has been rare, but remains a recognised risk with any injection.
Because PAAG is non-biodegradable, any complication — should it occur — may be more complex to manage than with short-acting injections.
This is discussed openly during consultation to ensure patients can make a fully informed decision about their care pathway.
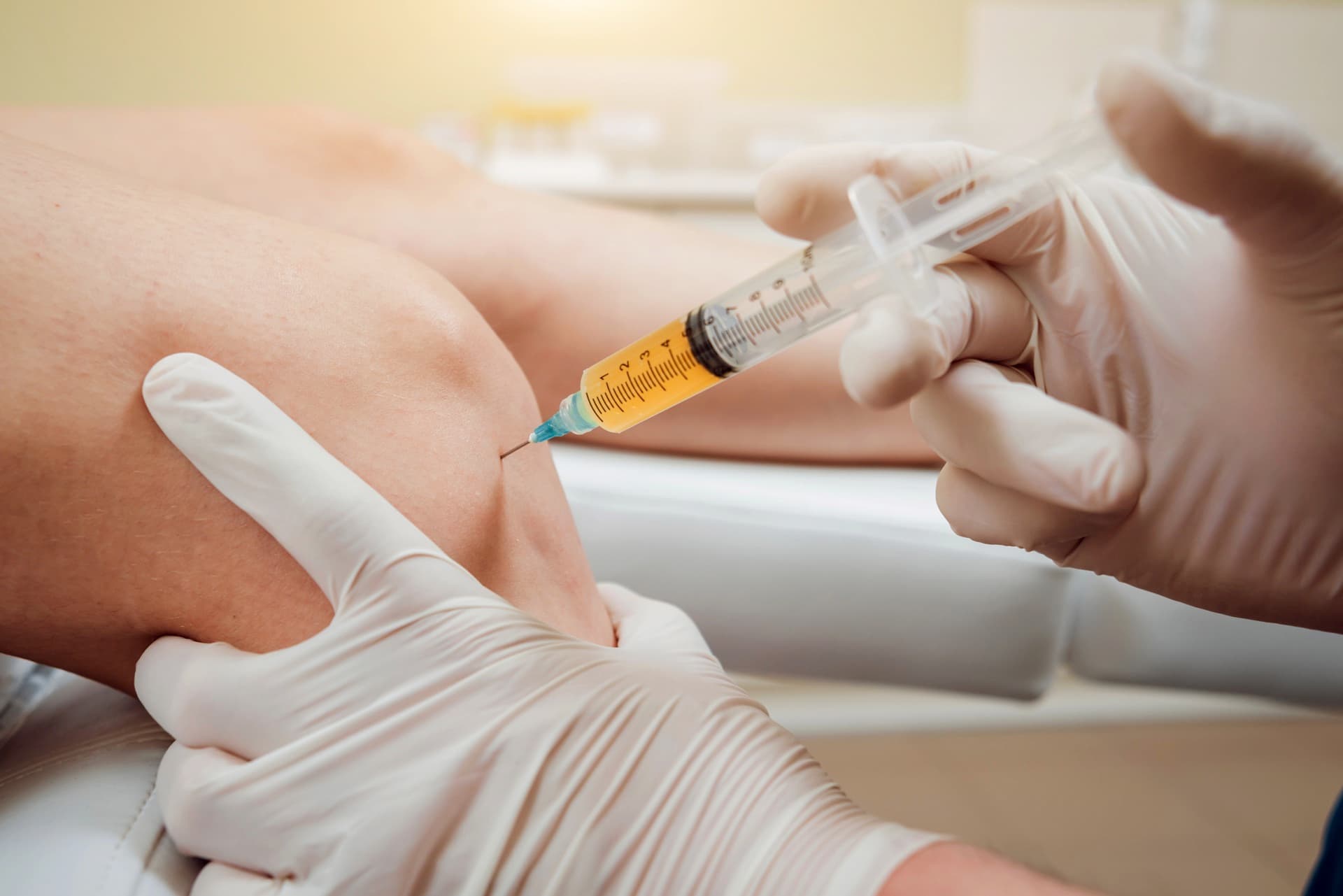
Why We Include This Warning
Integrity in patient care requires full disclosure. We believe patients should:
- Be aware that not all published PAAG data relates to knee osteoarthritis
- Understand that long-term data continues to evolve
- Have access to both supportive and critical literature
- Make decisions based on balanced information, not marketing
"For this reason, Arthrosamid® is not offered routinely, and we do not recommend it where the risk–benefit balance is unfavourable."
What This Means for You
If you are considering Arthrosamid®, our clinical team will guide you through a comprehensive consultation covering:
Potential benefits
Known and theoretical risks
Alternative treatments
Whether this option fits your goals and your knee condition
If you would like to read the published paper discussing PAAG (Aquamid) complications in more detail, please let us know.
Informed decisions require full information — including uncomfortable parts.

Book an Arthrosamid® Assessment – London
Long-lasting relief starts with the right decision — not just an injection.
Learn More about Arthrosamid
Deep dive into our clinical resources and patient guides.
Clinical Evidence
Evidence from Real Clinical Practice
Infection Safety
Infection Is a Serious Consideration With PAAG
Safety & Context
Arthrosamid Safety, Complications & Important Context
Full FAQ
Arthrosamid® (PAAG) Injection – Full Patient FAQ
Funding & Insurance
Funding, Insurance & NHS Coverage – Important Information
Patient Assessment
PAAG-8+ Questionnaire
Arthrosamid® (PAAG) Injection
Comprehensive Overview
Arthrosamid® is a registered trademark of Contura A/S. London Cartilage Clinic is not affiliated with or endorsed by Contura A/S.