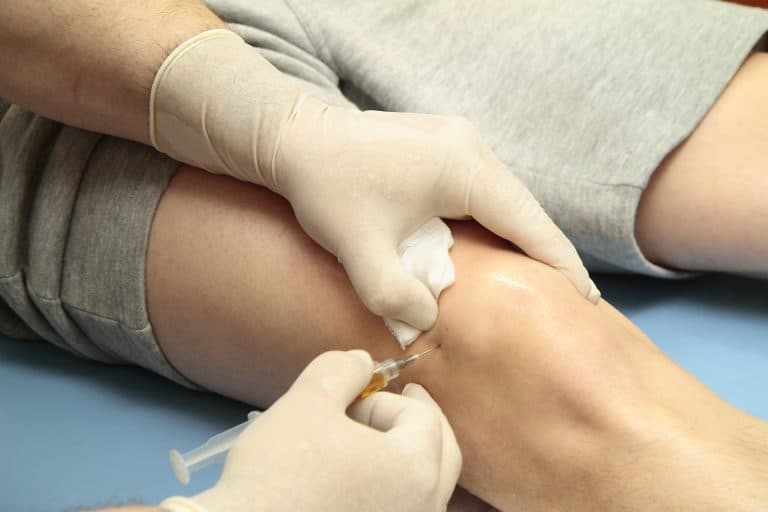
Arthrosamid® (PAAG)
Injection – Full Patient FAQ
Discover how this innovative non-biodegradable hydrogel provides long-lasting relief for knee osteoarthritis, combining cutting-edge medical technology with proven clinical safety.
Arthrosamid® (a registered trademark of Contura A/S.).
Patient Frequently Asked Questions
Comprehensive guide to Arthrosamid® treatment, infection protocols, and our independent clinical approach.
What is Arthrosamid®?
Arthrosamid® is a long-acting injection used to manage symptoms of knee osteoarthritis.
It is made from polyacrylamide hydrogel (PAAG) — a non-biodegradable, biocompatible material that remains inside the knee joint, acting as a long-term cushion and lubricant.
How is Arthrosamid® different from steroid or hyaluronic acid injections?
Most injections are absorbed over weeks or months.
Arthrosamid®:
- Is not absorbed
- Remains in the joint long-term
- Can provide symptom improvement lasting many months to up to two years in selected patients
Because it is permanent, patient selection, technique, and safety protocols matter more than with routine injections.
Does Arthrosamid® regenerate cartilage?
No.
Arthrosamid® does not regenerate cartilage and is not a stem cell treatment.
It helps by:
- Improving joint lubrication
- Reducing mechanical stress
- Improving pain and function
It is a joint-preserving, symptom-modifying treatment, not a cure.
Who is Arthrosamid® most likely to help?
Based on our independent clinical study with up to 24-month follow-up, benefit is more likely if:
- You are older
- Your arthritis is mild to moderate, not advanced bone-on-bone
- You do not have diabetes
- You receive bilateral knee injections (if both knees are symptomatic)
- You are seeking improvement, not perfection
- Your goal is to delay surgery, not necessarily avoid it forever
We assess this using a structured PAAG-8+ patient selection framework.
Who may benefit less?
Arthrosamid® may be less suitable if:
- You have advanced end-stage arthritis
- The knee is severely unstable or deformed
- You are already close to needing knee replacement
- You expect the injection to fully cure your knee
In these situations, benefit may be limited or temporary.
What does your clinical study involve?
Our Arthrosamid® data comes from an independent, clinician-led clinical study, not sponsored by the manufacturer.
- 269 patients
- 314 knees treated
- Prospective follow-up
- Up to 24 months
- Pain, function, complications, and progression to knee replacement recorded
This allows us to give real-world, balanced advice, rather than relying on marketing claims.
I’ve read about Aquamid complications — is that relevant?
Aquamid and Arthrosamid® are both PAAG-based products.
There are published papers describing PAAG complications, mainly involving:
- Cosmetic or soft-tissue injections
- Large volumes
- Non-joint tissues
- Long-term delayed presentations
These are not directly comparable to modern, image-guided knee injections — but they are important context, and we encourage informed patients to read the literature if they wish.
Is infection a risk with Arthrosamid®?
Infection is a risk with any joint injection, but with PAAG it carries greater significance because the material is permanent.
If infection occurs:
- It may be harder to eradicate
- Treatment may be prolonged
- Surgical intervention may occasionally be required
This is why our protocol places exceptional emphasis on infection prevention.
Why do you use intravenous (IV) antibiotics?
From the very beginning of Arthrosamid® use in the UK, Professor Paul Y.F. Lee adopted a deliberately conservative infection-prevention strategy.
- Professor Lee was the first clinician to perform Arthrosamid® knee injections in the UK
- He has personally performed 600+ Arthrosamid® injections
- His wider clinical group has performed over 2,000 injections
- All Arthrosamid® injections are performed with IV antibiotics
Why not oral antibiotics?
Our clinic does not recommend oral antibiotics for Arthrosamid® injections because:
- Oral absorption is variable
- Joint concentrations are unpredictable
- Timing around injection is less reliable
- Effectiveness against biofilm-forming bacteria may be reduced
IV antibiotics provide predictable, immediate protection at the time of injection — which matters with a permanent material.
Do you use anything additional during the injection?
Yes.
As part of Professor Lee’s specialist protocol, Arthrosamid® injections include a bespoke injection “protocol”, used alongside the PAAG.
This has been developed and refined based on:
- Scientific principles
- Large-volume clinical experience
- Ongoing review of outcomes and complications
Over time, this approach has been associated with a lower observed complication rate in our own practice.
What is in the injection protocol?
The exact composition and method form part of our internal protocol and are not shared externally.
This protocol reflects:
- Experience from 600+ injections by Professor Lee
- Learning from 2,000+ injections across the group
- Iterative refinement based on outcomes, not manufacturer guidance
Patients do not need to know the technical formulation to understand the purpose, rationale, and safety principles, which are discussed during consultation.
Is this protocol used everywhere?
No.
There is no single universal protocol for Arthrosamid®.
Some clinics use:
- Oral antibiotics
- No antibiotics
- Minimal sterile preparation
- Manufacturer-only guidance
Our approach is deliberately more cautious, reflecting experience with permanent materials and long-term outcomes.
What does this mean for me as a patient?
It means:
- Arthrosamid® is treated as a specialist procedure, not a routine injection
- Safety is prioritised over convenience
- Decisions are based on experience, science, and outcomes
- If you are uncomfortable with IV antibiotics or a bespoke protocol, Arthrosamid® may not be the right option
Why We Don’t Blindly Follow Manufacturer-Only Protocols
Manufacturers provide baseline guidance and suggest the use of oral anti-biotics designed to be widely applicable. That does not mean it is optimal for every clinical setting.
Our approach differs because:
- Arthrosamid® is non-biodegradable
- Complications, if they occur, can be more complex
- Published PAAG literature shows that context and technique matter
- Long-term outcomes depend on more than the product alone
For these reasons, Professor Paul Y.F. Lee developed a protocol based on:
- Direct clinical experience
- Large-volume outcome review
- Conservative risk management and audit data
- Continuous refinement over time
This includes:
- Mandatory IV antibiotics
- Enhanced sterile technique
- A bespoke injection protocol
- Structured follow-up
Still have questions about our specialized PAAG protocol?

Key Takeaway
Arthrosamid® is a permanent joint treatment.
At our clinic it is delivered using a bespoke, experience-led protocol, refined through thousands of injections and grounded in science — not marketing.

Why Independence Matters – Patient FAQ
Why does it matter that your Arthrosamid® work is independent?
Independence means that the clinical study and follow-up were led by clinicians, not by the manufacturer of Arthrosamid®.
This helps ensure that the information used to guide your treatment reflects real clinical experience, rather than marketing claims.
Was the Arthrosamid® study sponsored by the company?
No.
The study was not sponsored, commissioned, or funded by the manufacturer.
This allows outcomes to be discussed openly — including:
- Patients who improve
- Patients who notice limited benefit
- Patients who later progress to knee replacement
Does independence change how Arthrosamid® is recommended?
Yes — in a positive way.
Because we are not linked to the product:
- Arthrosamid® is not offered routinely
- It is recommended only when it is likely to help
- Other options are discussed honestly when appropriate
If Arthrosamid® is unlikely to provide meaningful benefit, we will say so.
Is Arthrosamid® still evidence-based if the study is independent?
Yes.
Independent clinical studies are commonly used to understand how treatments perform in everyday practice, outside of controlled or promotional settings.
This helps patients understand:
- How long benefits may last
- Who tends to do better
- When alternative treatments may be more appropriate
What does this mean for me as a patient?
It means your treatment plan is based on:
- Balanced clinical experience
- Clear discussion of benefits and limitations
- Realistic expectations
Independence supports honest advice — and better decisions.
Still have questions about our specialized PAAG protocol?

Book an Arthrosamid® Assessment – London
Long-lasting relief starts with the right decision — not just an injection.
Learn More about Arthrosamid
Deep dive into our clinical resources and patient guides.
Clinical Evidence
Evidence from Real Clinical Practice
Infection Safety
Infection Is a Serious Consideration With PAAG
Safety & Context
Arthrosamid Safety, Complications & Important Context
Full FAQ
Arthrosamid® (PAAG) Injection – Full Patient FAQ
Funding & Insurance
Funding, Insurance & NHS Coverage – Important Information
Patient Assessment
PAAG-8+ Questionnaire
Arthrosamid® (PAAG) Injection
Comprehensive Overview
Arthrosamid® is a registered trademark of Contura A/S. London Cartilage Clinic is not affiliated with or endorsed by Contura A/S.